methyl acetate formation
Methyl acetate MeAc esterification process by Eastman Chemical Company Agreda et al 1990. The optimum conditions for synthesis of acetic anhydride by carbonylation of methyl acetate have been found.

Methyl Acetate An Overview Sciencedirect Topics
Switch to calorie-based units.

. In this article we will discuss the methyl acetate formula its preparation etc. Information on this page. Use condensed structural formulas to write the equation for the formation of methyl acetate.
It is very flammable with a flashpoint of -10 C and a flammability rating. Siirola 1996 is a classic application of process intensification PI where the conventional MeAc production process consisting of one reactor and nine distillation columns was replaced by a single RD column. It is a clear colourless liquid that has a typical ester odour similar to glues and nail polish removers.
The incorporation of CO into the methoxy group leads to the formation of an acetyl intermediate. O CH3-C-OH CH3-OH- Previous questionNext question COMPANY About Chegg Chegg For Good College Marketing Corporate Development Investor Relations Jobs Join Our Affiliate Program Media Center Site Map LEGAL POLICIES Advertising Choices Cookie Notice. Methyl formate 109 is converted into AcOH under CO pressure in the presence of Lil and Pd OAc2 95.
Afterward the methoxide inter- mediate attacks a carbonyl group of the anhydride forming methyl acetate and the methylcarbonate ion Figure. What is methyl acetate used for. Methyl acetate is produced industrially by liquid phase reaction of acetic acid and methanol in presence of an acid catalyst.
And it may be a non-polar to weakly polar solvent. C 3 H 6 O 2. C 3 H 6 O 2.
Methyl acetate is produced by esterifying methanol with acetic acid in the presence of an esterification catalyst and separating the products in the. It is present in apple grapes banana and other fruits. Methyl acetate is formed due to the interaction between a dimethyl ether molecule and an acetyl intermediate.
Acetic acid methyl ester. Methyl acetate also known as methyl ethanoate acetic acid methyl ester MeOAc Tereton Devoton is a carboxylate ester with a molecular formula of C 3 H 6 O 2. Methyl acetate is produced industrially via the carbonylation of methanol as a byproduct of the production of acetic acidmethyl acetate also arises by esterification of acetic acid with methanol in the presence of strong acids such as sulfuric acid this production process is famous because of eastman kodaks intensified process using a reactive.
A reaction mechanism has been proposed for the carbonylation of methyl acetate with formation of acetic anhydride in the presence of a catalytic system consisting of rhodium III chloride zinc acetate and methyl iodide. Also the direct use of MA in coatings metal industries plastics automobile industries printing solvents and dyes causes the accumulation of MA in the atmosphere through evaporation 8 9 10. When acetic acid and methanol it forms methyl acetate and water Ch3COOHCH3OH--CH3C2H3O2H20.
The reaction is facilitated by the confinement effect characteristic of small zeolite pores in particular eight-membered pockets of mordenite. Copy Sheet of paper on top of another sheet. It has a pleasant odour and fleeting like the taste.
The net reaction is the formation of acetaldehyde from MeOH CO and H2P4. Methyl acetate MA is an important VOC that originates from degradation of methyl tert-butyl ether MTBE tert amyl ether TAME and ethyl tert-butyl ether ETBE. C 3 H 7 O 2 Molecular weight.
The Pd- catalyzed reductive carbonylation of methyl acetate with CO and H2 affords acetaldehyde. Empirical formula Hills system for organic substances. What is the equation for formation of methyl acetate.
It is a colourless volatile liquid. Methyl acetate is a carboxylate ester. Methyl chloroacetate C3H5ClO2 CID 7295 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.

Difference Between Methyl Acetate And Ethyl Acetate Compare The Difference Between Similar Terms
Synthesis Of Phenylmethoxycarbonylamino Methyl Acetate Via Ester Formation From Carboxylic Acid Chemsink
Ethyl Acetate Molecule Of The Month March 2003 Html Version

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram

Mass Spectrum Of Methyl Ethanoate C3h6o2 Ch3cooch3 Fragmentation Pattern Of M Z M E Ions For Analysis

Chemical Derivatization Of Acetate Using Methyl Chloroformate Mcf Download Scientific Diagram

Methyl Acetate Metac 99 5 Solvents Wacker Chemie Ag
What Is Reaction Of Methanol And Ethanoic Acid Quora
File Synthesis Of Methyl Acetate Svg Wikimedia Commons
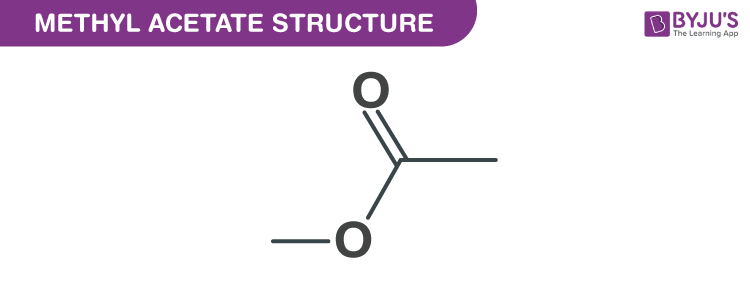
Methyl Acetate Structure Properties And Uses Of C3h6o2

Methyl Acetate Formula C3h6o2 Properties Uses Molar Mass Embibe

Chemical Derivatization Of Acetate Using Methyl Chloroformate Mcf Download Scientific Diagram

File Synthesis Of Methyl Acetate Svg Wikimedia Commons

Methyl Acetate An Overview Sciencedirect Topics

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram
Ethyl Acetate Molecule Of The Month March 2003 Html Version
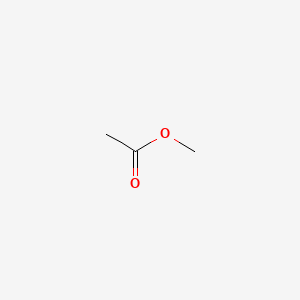
Methyl Acetate Ch3cooch3 Pubchem
What Smell Can I Get When Mixing Methanol With Acetic Ethanoic Acid Quora




0 Response to "methyl acetate formation"
Post a Comment